Solutions
The unique formula
BioConnection offers young and innovative biopharmaceutical companies access to our state-of-the-art GMP manufacturing facility and a broad range of support services. We are a contract services and manufacturing organization for the development and manufacture (Filling and Freeze drying) of injectable (bio)pharmaceutical products. The Netherlands are located centrally in Europe and from our proprietary site in Oss we supply both clinical as commercial products to our worldwide customers. Besides the central location of the Netherlands, the Dutch are also well known for their professional flexibility, organizational savvy and command of foreign languages. Besides our own EMA/FDA approved facility we have access to various facilities across Europe to enable an unmatched flexibility in solutions, capacity and capability. The cornerstones of our CMC services are: Drug Product development, Fill and Finish, Freeze drying and Finished Product services.

Your One Stop Shop
Our building blocks to fulfill your needs
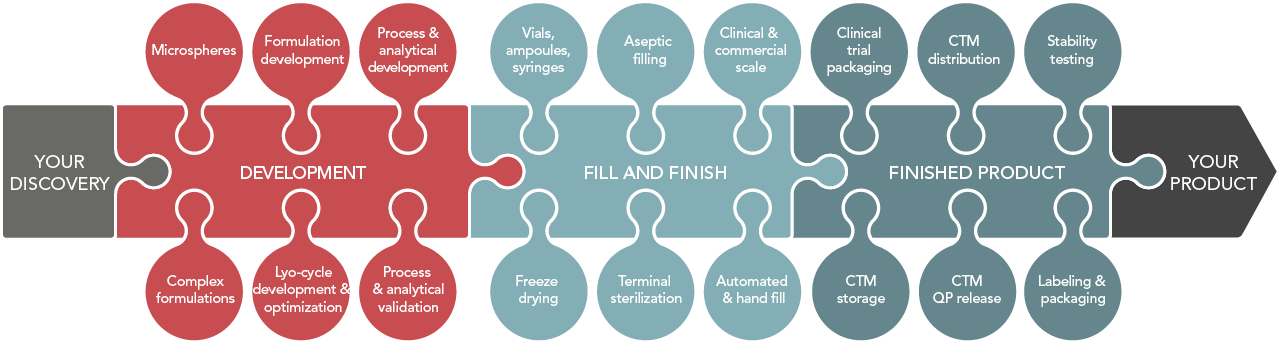
We have the means and expertise to bring your invention from the lab bench to the clinic and/or to the market. Due to our state-of-the-art production facility in Oss and our European network of development and manufacturing partners, we are able to provide you with tailor-made solutions and unchallenged flexibility. Our approach ensures a seamless development and manufacturing chain for your product.
BioConnection has an established network of suppliers in the biopharmaceutical sector. Therefore, we can help you find the service, products and knowledge you’re looking for in an unmatched dedicated and flexible environment.
Our expertise ranges from small molecules up to advanced products like proteins, vaccins, peptides, antibodies and complex formulations. Whether you need expertise on product development, business development or strategic advice, BioConnection saves you a great deal of time and money. It doesn’t matter whether you’re a startup or global size company, we work for all kinds of pharmaceutical enterprises.
Areas of Expertise
BioConnection offers expertise -always including direct personal attention from skilled operators- in various fields such as:
- Drug Product development
- Fill and Finish
- Freeze drying
- Clinical trial material services
- Project management
- Consultancy