Fill and Finish
BioConnection offers clinical and commercial Fill and Finish for your products. Our capabilities include liquid fill or lyophilization intended for vials, prefilled syringes or blowfill seals. Fill and Finish is the core of our company!
Clinical scale manufacturing
Our cornerstone is the clinical scale manufacturing plant in Oss. Via this plant, that complies with EMA and FDA regulations, we can provide you with the automated fill of clinical grade cGMP products in vials and syringes, both liquid and freeze-dried. Thanks to our network, we can also provide you with the handfill of vials, syringes and Fill and Finish almost any other container format, leading to a very flexible approach. Due to the efforts of BioConnection we can provide you a seamless transfer from the handfill unit to the automated unit.
Commercial scale-up of your product
We can also facilitate your commercial launch from this plant. Further commercial scale-up and manufacturing beyond our in-house scale is arranged through our network or we can transfer to your preferred supplier. You have total flexibility and control regarding the process, enabling a strategic fit between our capabilities and your wishes.
Please see the summary of our services below to view our capacities per vial, ampoule, syringe. We can’t wait to work with you!
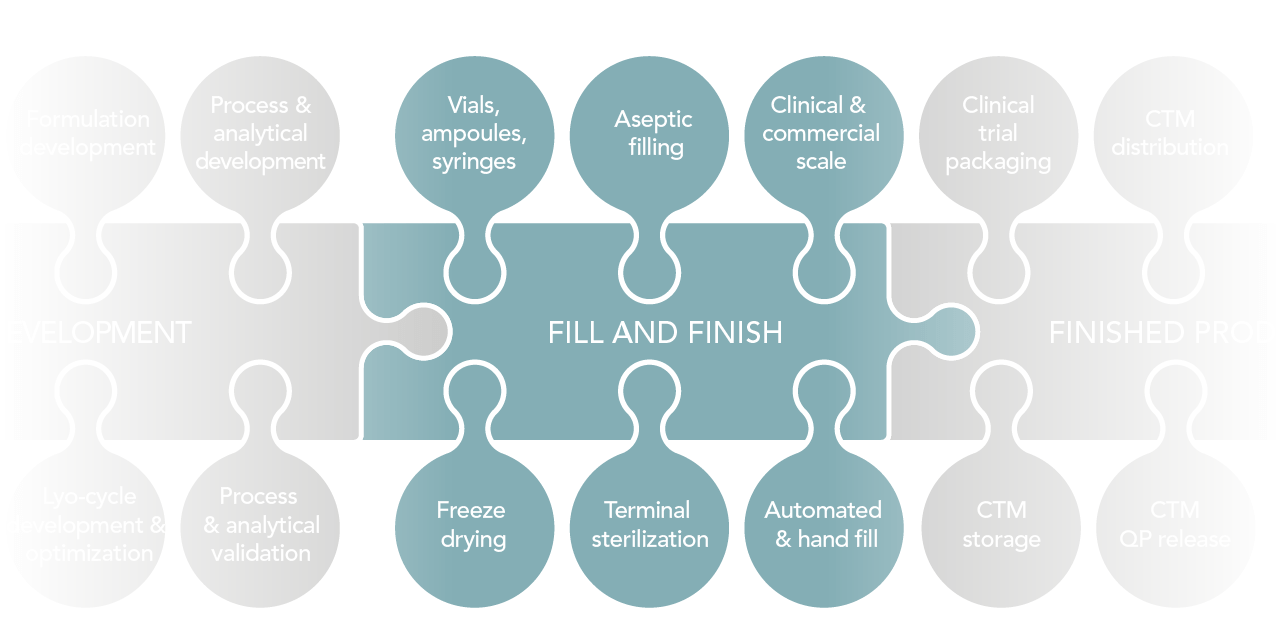
Summary of our services
Drug product development
- Formulation development
- Process and analytical development & validation
- Lyo-cycle development and/or optimization
- Transfer and scale-up to clinic
- Transfer and scale-up to market
Freeze drying
- Shelf areas ranging from 3 m² up to 10 m²
Clinical trial material services
- Labeling
- Clinical trial packaging
- QP release
- Storage
Fill and Finish
- Batch sizes: from 1 unit up to 150,000 units per batch
- Clinical and commercial scale
- Automated fill and hand fill
- Aseptic filling
- Vials: from 2 RDIN up to 50 RDIN
- Syringes: from 0.5 ml up to 10 ml
- Other primary packs: via handfill
- Terminal sterilization