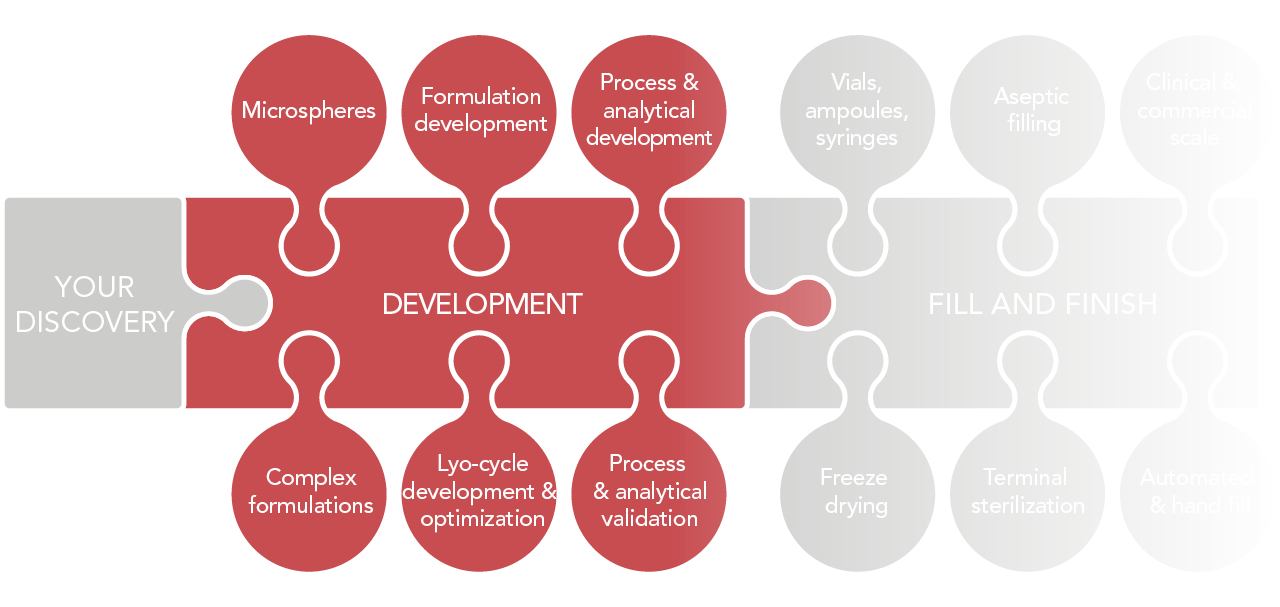
Development
- Formulation development (including Complex Formulations)
- Process development & validation
- Lyo-cycle development and/or optimization
- Analytical development & validation
- Transfer and scale-up
When you’re developing a finished dosage form, your project costs are most likely in a sound competition with your available funds. This is why choosing a CDMO is an important and long term decision. BioConnection can help you out with an oversight of the technology transfer and per-unit manufacturing cost. Let us also assist you with determining the often hidden costs: depending upon the mutual locations, you may incur costs for transportation, customs, duties, taxes and QP reviews. And worse, flaws in your (process) development could lead to mistakes, and even manufacturing problems. A functional relationship with your CDMO may prevent you to prevent costly do-overs. We try to help out by a dedicated Program Manager for each customer, providing a minimum ongoing oversight from your own. This way, you and your staff will remain available for other important activities.
BioConnection has a wide range of expertise in small molecules up to advanced products like proteins, vaccines, peptides and antibodies. We have worked, and are working, for a variety of customers. Our customers are located worldwide and range from small biotech startups up to large pharmaceutical companies. We have developed liquid and freeze-dried products and a variety of Complex Formulations including microspheres, nanoparticles and dispersions and emulsions. Also Medical Device challenges can be addressed, and we are now also capable of servicing you with your ATMP projects.
Flexibility and responsibility
Due to our flexible model we can provide you with almost the whole range of modern formulations. Together with our business partners we are able to develop the right formulation and via our network we have always the right connection for you. BioConnection acts as the linking pin in this network enabling the best tailor-made solution with unchallenged flexibility. You can either do the integration of your required building blocks yourselves, or let BioConnection provide you with a ‘Seamless Integration’.
We understand that development is never easy and that challenges will be faced. But we also understand that you need a partner you will take responsibility and works shoulder-to-shoulder with you to reach the desired endpoint: a successful clinical trial or market introduction. Due to the size, experience and attitude of BioConnection we are sure we are your perfect choice.
Summary of our services
Drug product development
- Formulation development
- Process and analytical development & validation
- Lyo-cycle development and/or optimization
- Transfer and scale-up to clinic
- Transfer and scale-up to market
Freeze drying
- Shelf areas ranging from 3 m² up to 10 m²
Clinical trial material services
- Labeling
- Clinical trial packaging
- QP release
- Storage
Fill and Finish
- Batch sizes: from 1 unit up to 150,000 units per batch
- Clinical and commercial scale
- Automated fill and hand fill
- Aseptic filling
- Vials: from 2 RDIN up to 50 RDIN
- Syringes: from 0.5 ml up to 10 ml
- Other primary packs: via handfill
- Terminal sterilization