Finished product
When your product leaves our facility, it needs a few additional steps in order to be ready for the clinic. These steps can also be provided by BioConnection. Depending on your needs and requirements, we can setup the further chain into the clinic. It’s a total package!

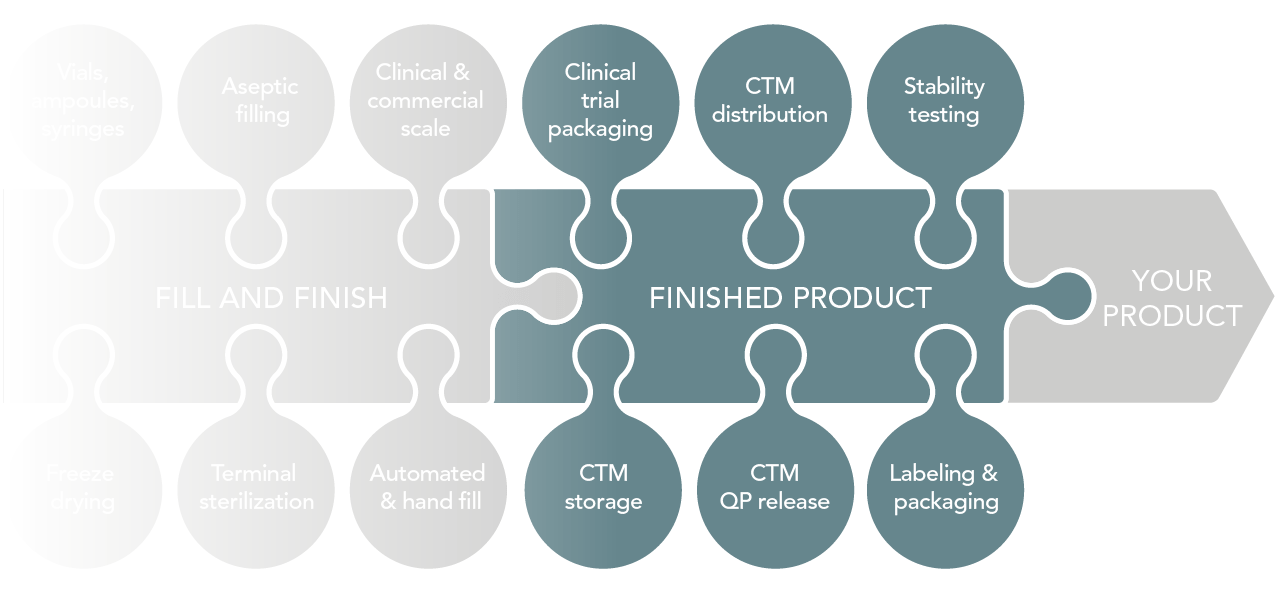
What we offer
- Labeling
- Clinical trial packaging
- QP release
- Stability testing
- Storage and distribution
The whole chain or a part: you are in control
By providing not only the manufacturing but also development and the follow-up services, we can bring your product from lab bench into the clinic. It’s as easy as that! But if you only need certain parts of this pallet of services, feel free to only use the ones you need. With BioConnection you are in control and you can make optimal use of our flexibility. Just contact us and make an appointment to talk about your drug product needs.
Summary of our services
Drug product development
- Formulation development
- Process and analytical development & validation
- Lyo-cycle development and/or optimization
- Transfer and scale-up to clinic
- Transfer and scale-up to market
Freeze drying
- Shelf areas ranging from 3 m² up to 10 m²
Clinical trial material services
- Labeling
- Clinical trial packaging
- QP release
- Storage
Fill and Finish
- Batch sizes: from 1 unit up to 150,000 units per batch
- Clinical and commercial scale
- Automated fill and hand fill
- Aseptic filling
- Vials: from 2 RDIN up to 50 RDIN
- Syringes: from 0.5 ml up to 10 ml
- Other primary packs: via handfill
- Terminal sterilization